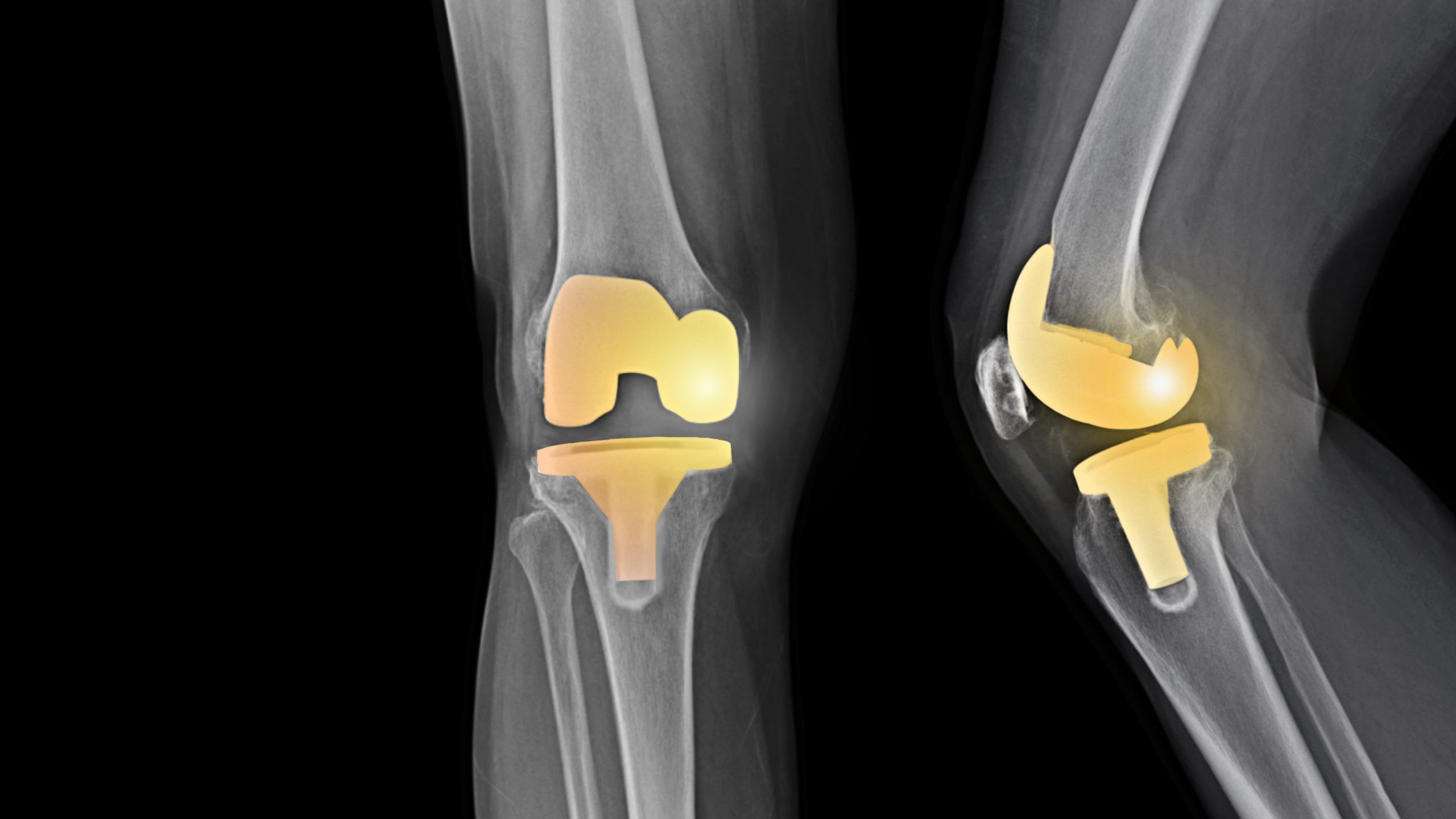
Exactech Inc., a medical device company headquartered in Gainesville, Florida, has recently recalled several models of hip and knee total joint replacement devices. Finding issues with their packaging, Exactech, Inc. provided information to implanting physicians and the FDA to initiate a Class II voluntary recall of these devices, which is defined by the FDA as, “a situation in which use of or exposure to a violative product [the Exactech, Inc.-manufactured total joint replacements] may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.” In this situation, as we discuss in the next section, the total joint replacement devices have been linked to bone degradation and death causing premature revision surgery, or surgery to replace the defective device.
Recall
Exactech, Inc., on June 29, 2021, issued a Class II voluntary recall of their hip replacement device, the Connexion GXL Acetabular Liner. Later, on February 7, 2022, Exactech, Inc. issued a Class II voluntary recall of their knee devices Optetrak, Optetrak Logic, and Truliant. For both recalls, the devices at issue were manufactured and implanted in 2004 or later and were packaged in an “out-of-specification” vacuum bag. The main issue in these recalls were the vacuum bags, which did not contain a secondary layer of ethylene vinyl alcohol (EVOH). The EVOH in medical device packaging gives additional oxygen resistance which reduces the device’s ability to oxidize. Because the packaging at issue did not contain the EVOH, the polyethylene inserts in these devices oxidized, resulting in premature wear.
Further Issues
Coupled with the lack of EVOH in packaging, the type of polyethylene liner used in these devices has been found to promote premature wear. Rather than a highly cross-linked polyethylene, which is the industry ‘norm’ and widely used for joint replacements, Exactech, Inc. has used a moderately cross-linked polyethylene. In the manufacturing process, cross-linking is done to strengthen polyethylene so that it can withstand higher amounts of pressure, whether from weight or activity, and ensure no particles will be rubbed off and absorbed into the neighboring bone. Highly cross-linked polyethylene is the strongest material, with moderately cross-linked polyethylene being slightly weaker. Combining the oxidation issue due to the packaging, which degrades the polyethylene, with the already weaker cross-linked polyethylene, the potential for device failure has skyrocketed.
Injuries
Because of the placement of these devices, they are subject to normal ‘wear and tear’. Our hip and knee joints bear a good amount of weight, regardless of full-body weight. With premature oxidation and wear of the polyethylene components in these devices, patients implanted with these defective devices have experienced early-onset:
- Osteolysis (bone degradation and death)
- Device loosening
- Pain
- Clicking and grinding
- Revision surgery
While some patients may only experience slight discomfort or even none at all, Exactech, Inc. notes in their own recall letters that there is a three to seven-fold increase in risk of these issues, including revision surgery, compared to other total joint replacements.
The medical device attorneys at The Simon Law Firm, P.C. are currently investigating cases against Exactech, Inc. for patients implanted with the devices at issue who received a revision surgery. If you or a loved one has received an Exactech, Inc. hip or knee replacement and underwent a revision surgery, please contact us today for a free, confidential consultation. When medical device companies place priority on profits rather than patients, The Simon Law Firm, P.C. will stand by those patients and fight for justice on their behalf.